Alzheimer’s disease research is crucial in understanding and combating one of the most challenging neurodegenerative diseases of our time. Scientists like Beth Stevens have changed the landscape of this field by focusing on microglial cells, which act as the brain’s immune system. These cells play a vital role by clearing out damaged neurons and supporting synaptic health, yet their malfunction can lead to devastating effects. Recent breakthroughs have revealed that improper pruning by microglia may contribute to Alzheimer’s, highlighting the significance of these cells in developing potential treatments and early detection biomarkers. As the number of individuals affected by Alzheimer’s is expected to rise dramatically in the coming years, ongoing research could pave the way for innovative therapies and improved quality of life for millions.
Research into Alzheimer’s disease, often termed dementia studies, explores the complexities of cognitive decline affecting countless individuals globally. Understanding the role of brain immune systems, particularly through the lens of microglial activity, offers fresh perspectives on treatment strategies for neurodegenerative disorders. By investigating pathways involving these immune cells, researchers can uncover new therapeutic options, inspired by prominent figures like neuroscientist Beth Stevens. This inquiry into how microglia manage synaptic health is vital as it sheds light on early intervention techniques and innovative medications. With the aging population, advancing this knowledge will be imperative for addressing the challenges posed by Alzheimer’s and similar conditions.
Understanding Microglial Cells: The Brain’s Immune System
Microglial cells are specialized immune cells located in the brain and spinal cord. Unlike other immune cells that circulate in the bloodstream, microglia are resident cells that play a crucial role in maintaining neuronal health. They constantly monitor the brain environment, identifying and responding to signs of injury or disease. When neurons are damaged or undergo pathological changes, microglial cells activate, clearing debris and facilitating recovery processes. Yet, this delicate balance can be disrupted, leading to neuroinflammation, a common feature across various neurodegenerative diseases, including Alzheimer’s.
Recent research, particularly by scientists like Beth Stevens, has revealed that improper activation of microglial cells can result in detrimental effects, contributing to the progression of Alzheimer’s and other neurodegenerative disorders. Studies suggest that aberrant microglial pruning of synapses may interfere with neural communication, exacerbating cognitive decline. As we deepen our understanding of these cellular behaviors, we move closer to developing targeted therapeutic strategies that modulate microglial activity, potentially alleviating symptoms of neurodegenerative diseases.
Beth Stevens and Her Transformative Research on Alzheimer’s Disease
Beth Stevens, a leading neuroscientist at Harvard, has dedicated her research to exploring the connection between microglial cells and neurodegenerative diseases such as Alzheimer’s. Her pioneering work has reshaped our understanding of how these immune cells influence brain health, particularly in the context of synaptic pruning. As Stevens delved into the intricacies of microglial function, she uncovered that while these cells are vital for brain maintenance, their dysfunction can lead to the acceleration of Alzheimer’s pathology. This inversion of knowledge not only deepens our insight into Alzheimer’s disease but also points to new avenues for potential treatments.
Stevens emphasizes the importance of curiosity-driven science in her research journey. With the necessary funding from the National Institutes of Health and other government bodies, she has been able to pursue groundbreaking studies that may one day inform Alzheimer’s treatment protocol. Her innovative approach underscores the significant role of basic science in unlocking the complex mechanisms of diseases. The potential insights gained from Stevens’ research could hold the key to earlier diagnosis and more effective therapies for the millions affected by Alzheimer’s.
The Role of Microglial Cells in Neurodegenerative Diseases
Microglial cells are not only crucial for maintaining brain homeostasis but also play a pivotal role in the development and progression of neurodegenerative diseases. In conditions such as Alzheimer’s disease, microglia become activated in response to amyloid beta plaques and tau tangles, initiating an inflammatory response that can become chronic and deleterious. This neuroinflammation is believed to exacerbate neuronal loss and cognitive decline, suggesting that targeted modulation of microglial activity may be a promising therapeutic strategy.
Innovative studies, like those conducted by Beth Stevens, have revealed the complex interplay between microglial responses and neurodegeneration. Stevens’ work illustrates how a better understanding of microglial biology can lead to the identification of novel therapeutic targets. By harnessing the innate abilities of microglial cells to prune dysfunctional synapses, researchers aim to restore healthy neural circuits disrupted in diseases like Alzheimer’s. This paradigm shift in understanding the role of microglia could significantly influence treatment plans in the future.
The Future of Alzheimer’s Treatment: Insights from Microglial Research
The future of Alzheimer’s treatment lies in innovative research focused on the roles of microglial cells. With an increasing number of individuals diagnosed with Alzheimer’s, the need for effective therapies has never been more urgent. Recent breakthroughs highlight the potential of small molecule drugs that can enhance or inhibit the activity of microglia, paving the way for personalized treatment strategies. By targeting the immune responses of these brain cells, researchers hope to mitigate the effects of neurodegeneration.
As the research progresses, the insights garnered from scientists like Beth Stevens could lead to the development of new biomarkers for early detection of Alzheimer’s disease. The ability to identify changes in microglial activity could facilitate timely interventions. Furthermore, integrating this knowledge into clinical practice may transform how we approach treatment for neurodegenerative diseases, offering hope to millions and potentially redefining the landscape of Alzheimer’s disease management.
The Importance of Early Diagnosis in Alzheimer’s Disease
Early diagnosis of Alzheimer’s disease is vital for implementing effective treatment plans and managing patient care. Research indicates that interventions are more effective when administered in the earlier stages of the disease, highlighting the necessity for better diagnostic markers. As scientists explore the role of microglial cells in Alzheimer’s pathology, their findings may lead to the identification of new biomarkers that make it possible to detect the disease long before significant cognitive decline occurs.
Moreover, timely diagnosis allows for the potential of lifestyle modifications and early therapeutic interventions that could slow the progression of Alzheimer’s. As awareness increases around the implications of microglial dysfunction, it becomes crucial to educate healthcare providers and the public alike on the benefits of early detection. By fostering a proactive approach to Alzheimer’s care, we can improve quality of life for individuals facing this challenging diagnosis.
Innovative Strategies for Alzheimer’s Disease Prevention
As the landscape of Alzheimer’s disease research continues to evolve, innovative strategies for prevention are gaining traction. Recent studies spotlight the role of lifestyle factors such as diet, exercise, and mental stimulation in potentially lowering the risk of Alzheimer’s. These preventative measures can reduce inflammation in the brain, essentially supporting the health of microglial cells, which play a protective role in maintaining cognitive function.
Additionally, multifaceted approaches involving community programs focused on education, social engagement, and health screenings will contribute to a holistic strategy for Alzheimer’s prevention. By emphasizing the need for brain health across all stages of life and fostering environments that promote cognitive well-being, we can create a society better equipped to tackle the impending Alzheimer’s crisis.
The Impact of Neuroinflammation on Brain Health
Neuroinflammation remains a focal point in understanding Alzheimer’s disease and other neurodegenerative conditions. The activation of microglial cells in response to neurotoxic insults leads to elevated inflammatory cytokines that can harm neuronal health. Research indicates that chronic neuroinflammation can accelerate the progression of Alzheimer’s disease, symptomatizing the importance of controlling inflammatory processes within the brain.
As studies evolve, efforts to manage neuroinflammation could result in novel therapeutic approaches that not only target the symptoms but also address the underlying biological mechanisms. Exploring the dual nature of microglial cells—both beneficial and detrimental—holds promise for developing strategies that control inflammation while preserving neuroprotection, thus enhancing overall brain health.
The Role of Federal Funding in Advancing Alzheimer’s Research
Federal funding plays a critical role in advancing Alzheimer’s research and supporting innovative scientists like Beth Stevens. Grants from agencies like the National Institutes of Health (NIH) have been instrumental in driving research initiatives focused on understanding complex diseases. Such financial backing allows early-career scientists to explore novel hypotheses, unlocking insights into the mechanisms underlying conditions like Alzheimer’s disease.
Moreover, sustained federal investment in Alzheimer’s research is paramount as the aging population grows. As the number of individuals affected by Alzheimer’s is projected to sharply rise, the allocation of funds towards this research ensures that breakthroughs can be translated into effective treatments. It highlights the urgent need for continued support to address these challenges and improve the lives of millions who are vulnerable to neurodegenerative diseases.
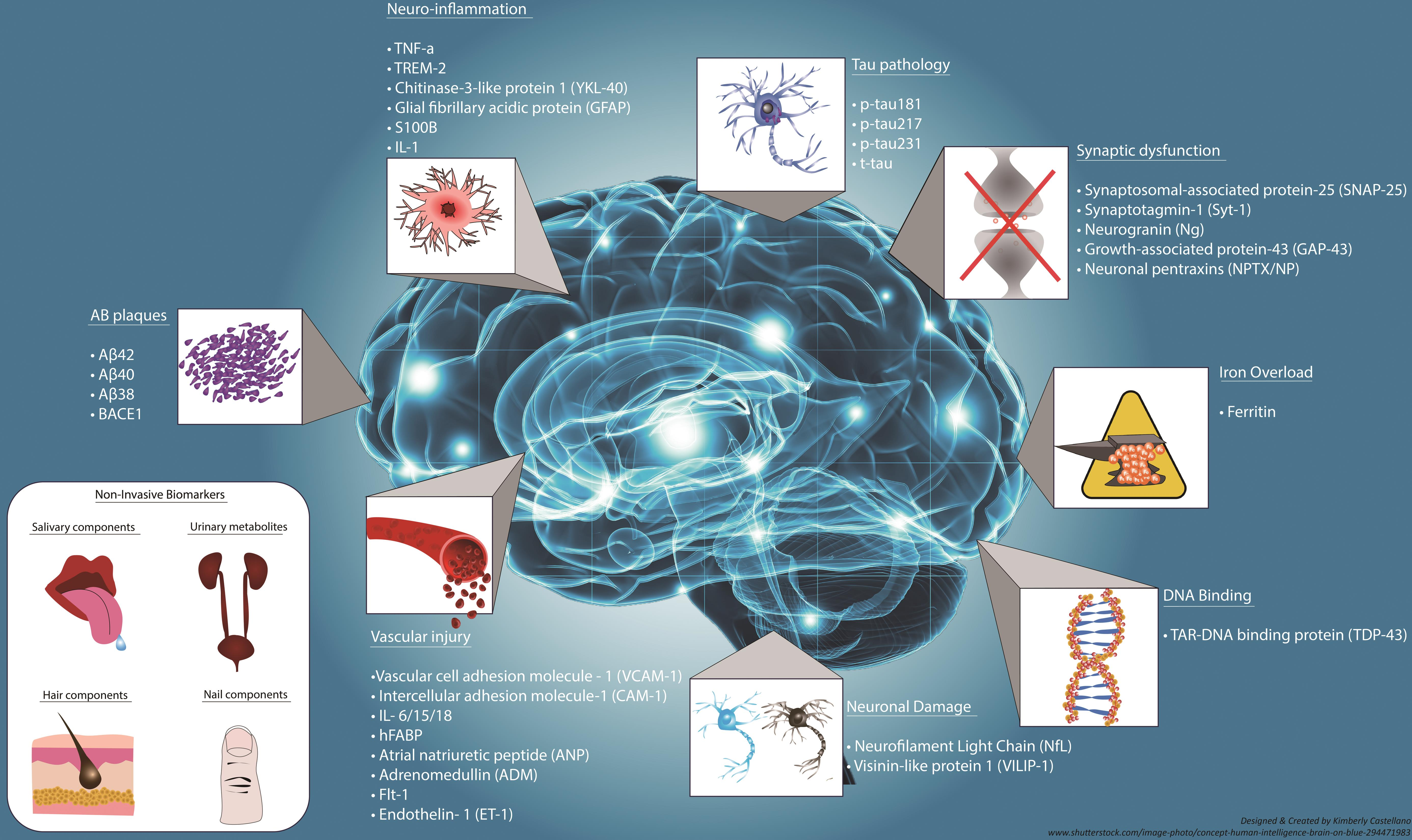
New Frontiers in Alzheimer’s Disease Biomarkers Discovery
The discovery of reliable biomarkers for Alzheimer’s disease is one of the most significant areas of research today. Identifying biological indicators that signal disease progression can facilitate earlier diagnosis and more targeted intervention strategies. With the advances in understanding microglial dysfunctions in Alzheimer’s, it has become clear that measuring inflammatory markers related to microglial activity can provide valuable insights into the disease’s timeline.
Additionally, partnerships between research institutions and biotech companies are paving the way for innovative diagnostic tools. By leveraging the latest technological advancements, scientists aim to develop blood-based or imaging biomarkers that make Alzheimer’s detection less invasive and more precise. These breakthroughs may eventually lead to individualized treatment strategies that improve patient outcomes across the board.
Frequently Asked Questions
How do microglial cells impact Alzheimer’s disease research?
Microglial cells play a crucial role in Alzheimer’s disease research as they act as the brain’s immune system, patrolling for damage and helping to clear out dead cells. Aberrant pruning by these cells has been linked to the development of neurodegenerative diseases, including Alzheimer’s. Research on microglia helps scientists understand disease mechanisms and develop targeted Alzheimer’s treatment strategies.
What are the contributions of Beth Stevens to Alzheimer’s disease research?
Beth Stevens has significantly contributed to Alzheimer’s disease research by transforming the understanding of microglial cells and their role in brain health. Her work has shown that improper pruning of synapses by microglia can lead to neurodegenerative diseases like Alzheimer’s. Her findings are foundational for developing new medicines and biomarkers that could improve the early detection and treatment of Alzheimer’s patients.
Why are microglial cells important in the study of neurodegenerative diseases like Alzheimer’s?
Microglial cells are essential in studying neurodegenerative diseases such as Alzheimer’s because they maintain brain health by clearing damaged cells and regulating synaptic pruning. Understanding their function is key to Alzheimer’s research, as malfunctioning microglia can lead to disease progression. This research helps identify potential therapeutic targets for effective Alzheimer’s treatment.
What role do neuroscience and microglial research play in advancing Alzheimer’s treatment?
Neuroscience and microglial research are pivotal in advancing Alzheimer’s treatment by illuminating the immune processes in the brain. Research conducted by scientists like Beth Stevens has revealed how microglial dysfunction can contribute to Alzheimer’s progression, paving the way for innovative therapies that could modify disease onset and improve patient outcomes.”},{
| Key Point | Details |
|---|---|
| Research Focus | Beth Stevens studies microglial cells, the brain’s immune system. |
| Importance of Microglia | Microglia help clear damaged cells and prune synapses, but abnormal function contributes to Alzheimer’s and other neurodegenerative diseases. |
| Research Foundation | Stevens’ work is supported by NIH and federal funding, emphasizing the role of basic science in advancing understanding of complex diseases. |
| Potential Impact | Stevens’ research may lead to new medicines and biomarkers for early detection of Alzheimer’s disease. |
| Projected Growth | The number of Alzheimer’s cases in the U.S. is expected to double by 2050, increasing care costs significantly. |
Summary
Alzheimer’s disease research is witnessing transformative breakthroughs led by scientists like Beth Stevens, who have reshaped our understanding of microglial cells in the brain. These immune cells play a crucial role in maintaining neural health, and Stevens’ findings underscore how their malfunction can influence neurodegenerative diseases. By emphasizing the importance of basic science, her work holds promise for developing effective treatments and enhancing early detection methods for Alzheimer’s. With the predicted rise in cases, such research efforts are vital to mitigating the future impact of this devastating condition.