Microglial research is at the forefront of neuroscientific exploration, offering crucial insights into the brain’s immune system and its role in neurodegenerative disorders such as Alzheimer’s disease. These specialized cells, known as microglia, are essential for maintaining neurological health by performing vital functions, including synaptic pruning—the process by which unnecessary synapses are eliminated. Pioneered by researchers like Beth Stevens, this field has evolved dramatically over the years, uncovering unexpected links between microglial activity and the pathology of diseases like Alzheimer’s and Huntington’s. Through their research, Stevens and her team have investigated how improper microglial functions can lead to harmful outcomes, ultimately shaping the development of potential therapies. This innovative work not only enhances our understanding of the brain but also holds promise for improving the lives of millions affected by neurodegenerative conditions.
The study of glial cells, particularly microglia, represents a significant advancement in our understanding of brain health and disease. These cells are integral to the central nervous system, serving as the brain’s first line of defense against injury and infection while also playing a critical role in the maintenance of neuronal connections. Investigations into how microglia manage synaptic pruning have revealed their complex interactions with neurons, highlighting their importance in various neurodegenerative disorders, including those associated with aging. The ongoing research led by experts, such as Beth Stevens, is reshaping the landscape of neurobiology by focusing on the connections between immune function and cognitive health. This fresh perspective not only targets the fundamental mechanisms underlying these disorders but also aims to develop new strategies for treatment and prevention.
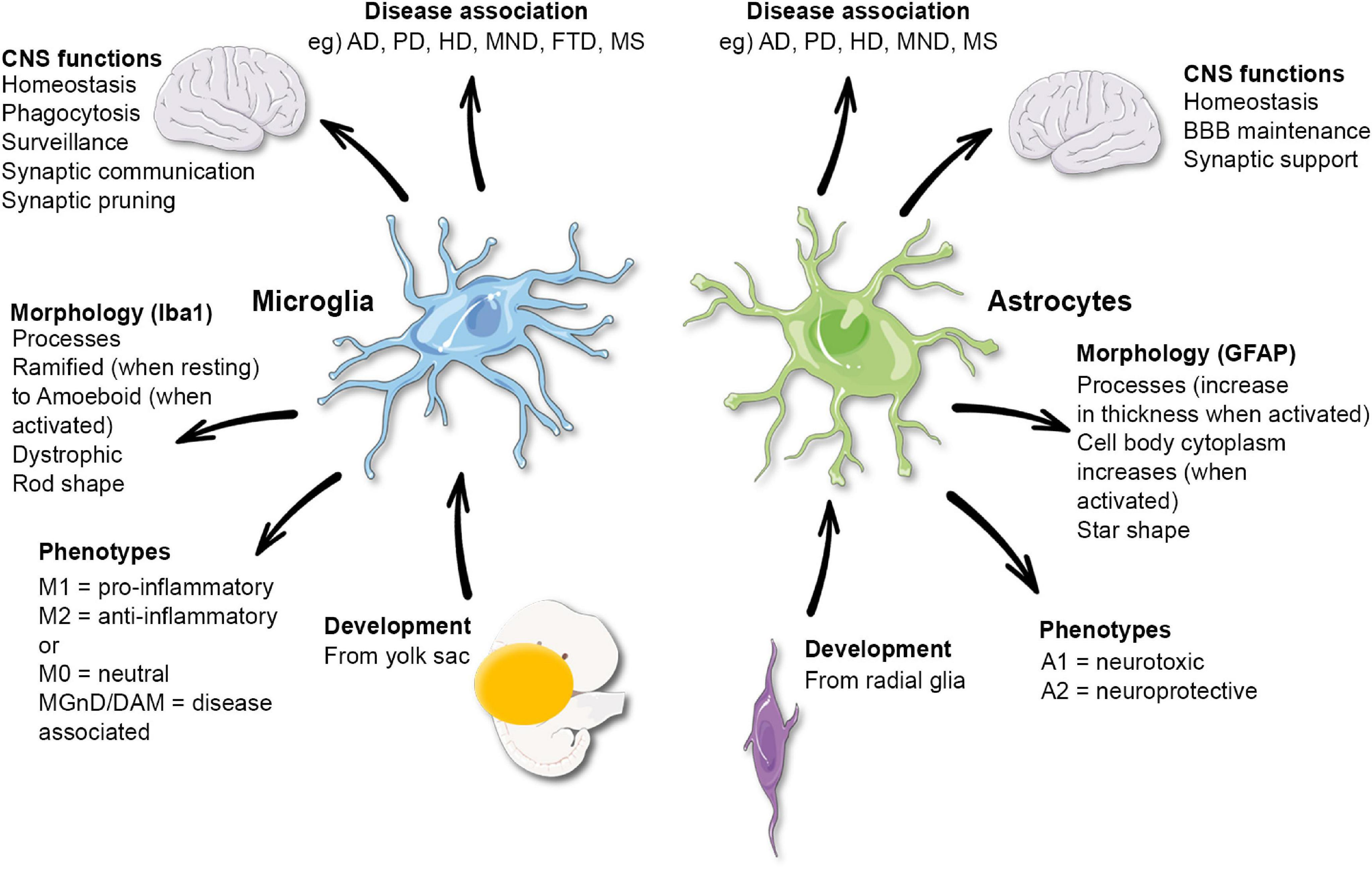
Understanding Microglia Functions in Neurodegenerative Disorders
Microglia are integral components of the brain’s immune system, playing essential roles in neurodevelopment and neuroprotection. Significantly, these cells monitor the brain environment, clearing away dead neurons and debris, a process critical for maintaining optimal synaptic health. This is particularly relevant in the context of neurodegenerative disorders such as Alzheimer’s disease, where the failure of microglial cells to perform these functions can exacerbate the progression of the disease. Aberrations in their function can lead to excessive synaptic pruning, contributing to cognitive decline in patients.
Research into microglial functions, particularly by scientists like Beth Stevens, reveals how these cells can shift from protectors to potential culprits in neurodegenerative diseases. For instance, Stevens’ work demonstrates that when microglia improperly prune synapses, it can lead to significant cognitive impairments. Such insights underscore the importance of understanding the dual role of microglia in both maintaining brain health and their involvement in pathological conditions like Alzheimer’s, ultimately paving the way for new therapeutic strategies.
The Role of Synaptic Pruning in Alzheimer’s Disease
Synaptic pruning is a natural process in which the brain eliminates weaker synaptic connections while strengthening others, essential for cognitive function and memory. However, in Alzheimer’s disease, this process can become dysregulated. Overactive microglial cells may over-prune synaptic connections, leading to neuronal loss and contributing to the manifestations of the disease. Stevens’ research highlights the delicate balance that microglia must strike between beneficial and harmful synaptic pruning, providing critical insights into disease mechanisms.
The implications of this research extend beyond basic science, offering potential pathways for developing biomarkers and therapeutic interventions for Alzheimer’s disease. By understanding how synaptic pruning operates within the context of neurodegeneration, scientists may identify new targets for drugs that can modulate microglial activity. Therefore, attending to the nuanced roles of synaptic pruning influenced by microglial cells is essential for anyone looking to combat neurodegenerative disorders.
Exploring the Impact of Beth Stevens’ Research on Alzheimer’s Care
Beth Stevens’ groundbreaking research has transformed our approach to understanding Alzheimer’s disease and other neurodegenerative disorders. By framing microglia as active players in synaptic health, her work has opened up new lines of inquiry that connect basic neuronal processes with disease outcomes. This shift in perspective emphasizes the need for continuous funding and support for basic science, as it can lead to significant advances in understanding and potentially mitigating the effects of diseases like Alzheimer’s.
The practical applications of Stevens’ findings are profound; they may lead to the identification of new biomarkers that can predict disease progression and therapeutic targets to enhance cognitive function in patients. Such advancements are crucial for the estimated 7 million Americans living with Alzheimer’s, illuminating the path toward more effective treatments and improved quality of life. Thus, Stevens’ contributions underscore the integral relationship between basic research and clinical advancements in neurodegenerative diseases.
Federal Support for Microglial Research
The trajectory of Beth Stevens’ research on microglia has been bolstered significantly by federal support, particularly through funding from the National Institutes of Health (NIH). This funding has been pivotal in allowing for the exploration of complex questions surrounding microglial behavior in various contexts, including normal development and disease. With the backing of federal resources, her lab has been able to conduct extensive studies that have fundamental implications for understanding neurodegenerative disorders.
Such support is vital for fostering curiosity-driven science, which can often lead to unexpected breakthroughs. As Stevens noted, the very foundation of her research was laid with federal funding, facilitating a line of inquiry that may have otherwise remained unexplored. The consistent investment in basic research is critical, as it fosters a scientific environment where innovative ideas can flourish, potentially transforming our understanding of neurodegenerative diseases.
Implications of Aberrant Microglial Activity
Aberrant microglial activity has significant implications for the progression of neurodegenerative diseases. Stevens’ research suggests that when microglial cells overreact to pathological signals, they may begin to eliminate necessary synaptic connections instead of targeting damaged neurons. This aberrant pruning not only contributes to neuronal cell death but also disrupts neural circuits essential for cognitive function, yielding detrimental effects for the brain.
Recognizing the signs of such dysregulation in microglial behavior could be key to developing interventions aimed at halting or reversing the effects of Alzheimer’s disease. By targeting the specific pathways involved in microglial activation and their effects on synaptic pruning, new therapies could be created to help restore brain health and functionality in affected individuals. Therefore, understanding microglial functions is critical for formulating new strategies against neurodegeneration.
New Biomarkers Emerging from Microglial Studies
As researchers delve deeper into the roles of microglia in neurodegenerative diseases, new biomarkers are emerging that could revolutionize the way we diagnose and treat conditions like Alzheimer’s disease. Beth Stevens’ investigations into microglial functions have highlighted specific markers associated with aberrant synaptic pruning, potentially allowing for earlier detection of disease processes before significant cognitive decline occurs. These biomarkers can provide insights into disease mechanisms and assist in monitoring disease progression.
Early diagnosis through these biomarkers can also pave the way for targeted therapeutic strategies that may halt or slow the neurodegenerative processes. The identification of such indicators is crucial for clinicians as it provides a clearer picture of a patient’s condition and could inform treatment pathways tailored to individual needs. Thus, microglial research not only enhances our understanding of diseases but also points toward practical tools for improving patient care.
The Importance of Curiosity-Driven Science in Neuroscience
Curiosity-driven science plays a pivotal role in advancing our understanding of complex biological systems, such as the brain’s immune responses. Researchers like Beth Stevens exemplify how following scientific curiosity can yield significant insights into conditions like Alzheimer’s disease. The unpredictability of basic research means that breakthroughs often arise from unexpected findings, and Stevens’ journey illustrates how fundamental questions about microglial behavior can have far-reaching implications for understanding neurodegenerative disorders.
Investing in curiosity-driven research not only fosters innovation but also enhances our ability to address challenging medical problems. As Stevens has highlighted, the foundation of her work is a testament to the power of exploration in science — her findings on microglial functions provide a roadmap for potential treatments that could alter the course of diseases affecting millions. Thus, promoting a culture of inquiry in the field of neuroscience is essential for translating basic research into viable clinical applications.
Looking Ahead: Future Directions in Microglial Research
Looking ahead, the field of microglial research presents numerous opportunities for novel discoveries in the treatment of neurodegenerative diseases. As scientists build on the foundational work done by investigators like Beth Stevens, future studies may elucidate the precise mechanisms by which microglia contribute to conditions such as Alzheimer’s disease. Understanding the nuances of microglial activation and their interactions with neural circuits will be critical in identifying new therapeutic targets.
Moreover, the potential for developing pharmacological agents that modulate microglial activity could lead to groundbreaking advances in clinical care. By finding ways to balance microglial functions to support neuronal health without triggering adverse effects, researchers hope to transform treatment paradigms for neurodegenerative disorders. As the body of evidence grows, so too does the hope for improved interventions that may one day enhance the lives of individuals living with Alzheimer’s disease and other related conditions.
Frequently Asked Questions
What role do microglia play in Alzheimer’s disease research?
Microglia serve as the brain’s immune cells, crucial for maintaining neural health. In Alzheimer’s disease research, studies have shown that these cells are involved in synaptic pruning—the process of removing unnecessary synapses. Aberrant microglial activity in this process can lead to increased neurodegenerative changes, making them vital targets for therapeutic interventions.
How does synaptic pruning by microglia relate to neurodegenerative disorders?
Synaptic pruning, primarily conducted by microglia, is essential for brain development and function. In neurodegenerative disorders like Alzheimer’s disease, improper pruning can remove healthy synapses or fail to clear dysfunctional ones, exacerbating cognitive decline. Research in this area is vital for understanding the pathological mechanisms of diseases.
What were the key discoveries of Beth Stevens in microglial research?
Beth Stevens’ research has fundamentally changed our understanding of microglia, especially their functions in synaptic pruning and their role in neurodegenerative disorders such as Alzheimer’s disease. Her investigations have revealed how microglial dysfunction can contribute to unhealthy brain environments and have led to the development of potential biomarkers and treatments.
How do microglial functions influence Alzheimer’s disease progression?
Microglial functions, including surveillance and synaptic pruning, significantly influence Alzheimer’s disease progression. Dysregulation in these processes can lead to neuroinflammation and neuronal damage, intensifying disease symptoms. Ongoing microglial research aims to identify how these mechanisms can be targeted for therapeutic strategies.
Why is understanding microglia important for neurodegenerative research?
Understanding microglia is crucial in neurodegenerative research because they play a pivotal role in the brain’s immune response. Their involvement in processes like synaptic pruning affects neuronal health and can lead to diseases such as Alzheimer’s if disrupted. Insights from microglial research can inform new treatment approaches and preventive strategies for these conditions.
What impact does microglial research have on developing Alzheimer’s treatments?
Microglial research, particularly studies led by scientists like Beth Stevens, provides insights into how these cells can be manipulated to restore normal synaptic pruning and prevent neurodegeneration. This research is essential for developing targeted therapies that could slow down or halt the progression of Alzheimer’s disease.
What funding sources support microglial research related to Alzheimer’s disease?
Much of the funding for microglial research, especially in the context of Alzheimer’s disease, comes from federal agencies like the National Institutes of Health (NIH). These funds facilitate exploratory studies that identify how microglial functions can be harnessed to combat neurodegenerative disorders.
How has basic science contributed to advancements in microglial research?
Basic science has been foundational for advancements in microglial research. By exploring neurodevelopment and synaptic functions in animal models, researchers have laid the groundwork for understanding microglial roles in conditions like Alzheimer’s disease, ultimately driving discoveries that lead to effective interventions.
| Key Point | Description |
|---|---|
| Microglial Cells | Act as the brain’s immune system, clearing out dead or damaged cells and pruning synapses. |
| Aberrant Pruning | Improper pruning by microglia can contribute to neurodegenerative diseases like Alzheimer’s and Huntington’s. |
| Research Foundation | Stevens credits NIH and federal funding for foundational research that enables advances in microglial research. |
| Importance of Basic Science | Basic science exploration in model organisms can lead to significant discoveries applicable to human health. |
| Future Implications | The discoveries in microglial research may lead to new biomarkers and therapies for diseases affecting millions. |
Summary
Microglial research is pivotal in understanding brain diseases, particularly Alzheimer’s. The ongoing exploration of microglial cells reveals their crucial role in brain immunity and synaptic maintenance, leading to new insights and potential treatments. Beth Stevens’ innovative work highlights the importance of foundational research, driven by curiosity and federal support, in developing strategies to tackle neurodegenerative diseases effectively. This foundational understanding is vital for improving the lives of the millions affected by Alzheimer’s and similar disorders.