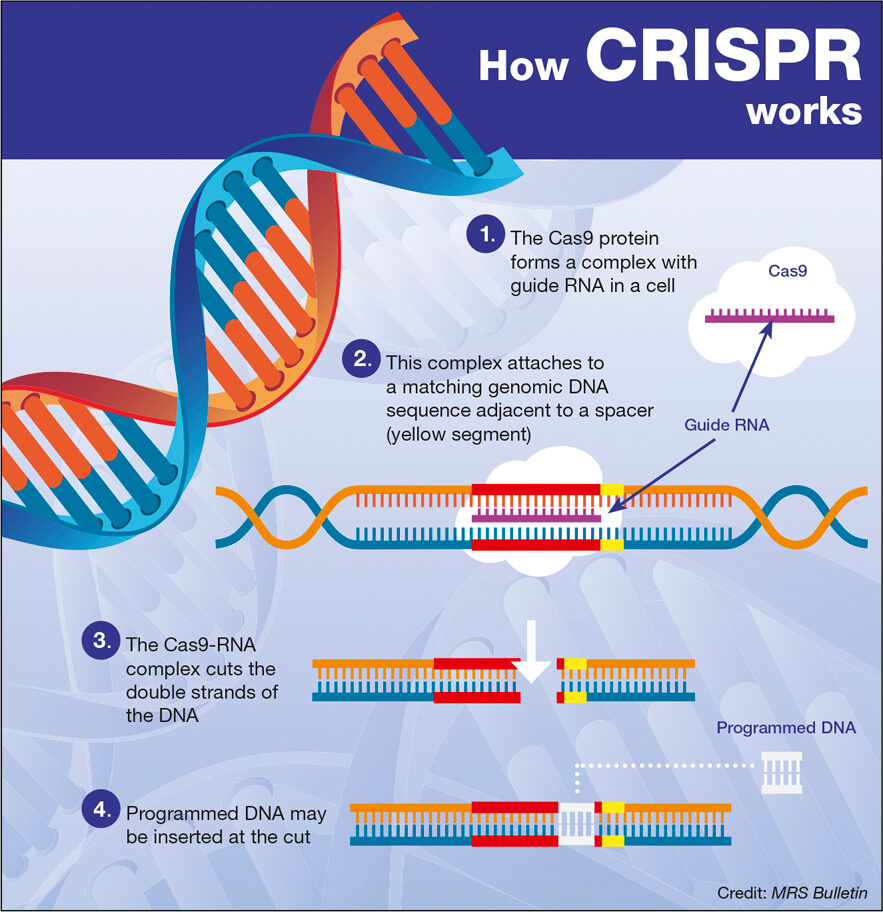
CRISPR gene editing has emerged as a revolutionary technology in the field of genetics, promising the potential to not only treat but also cure life-threatening diseases such as sickle cell disease. This innovative technique allows scientists to precisely edit DNA, igniting a passionate debate surrounding gene editing ethics and the implications of genetic modification on health equity. While the ability to eradicate debilitating conditions brings hope, it also raises significant concerns regarding germline editing and the moral questions it provokes. As discussions around the risks of genetic manipulation unfold, society grapples with the potential costs tied to these advancements—both monetary and ethical. The intersection of CRISPR technology and medical ethics continues to challenge our perceptions of what it means to be human.
Gene editing through the CRISPR mechanism has introduced a new era in biotechnology, enabling researchers to alter genetic sequences with unprecedented accuracy. This transformative method has opened up avenues for curing chronic inherited conditions, yet it also raises alarms regarding the ethical landscape of genetic alterations. As debates about the responsibilities of scientists in modifying human traits intensify, the risks associated with genetic modification come into sharp focus. The implications of adjusting genes, particularly in germline cells, trigger concerns about societal equity and fairness. Ultimately, as we navigate this groundbreaking technology, we must grapple with the balance of innovation and ethical responsibility.
The Promise of CRISPR Gene Editing
CRISPR gene editing represents a transformative frontier in modern medicine, with the potential to revolutionize treatments for genetic disorders. One of the most prominent applications; the ability to cure diseases like sickle cell anemia, has ignited hope among patients and healthcare providers alike. By targeting the root causes of genetic illnesses, CRISPR allows for precise alterations to DNA, offering a permanent solution rather than symptomatic relief. However, this promise comes with complex ethical dilemmas that cannot be overlooked.
Neal Baer’s assertion that ‘we can cure sickle cell’ raises essential questions about the implications of such capabilities. The technology essentially enhances our ability to address not only health anomalies but also broader genetic modifications, potentially leading to changes that extend beyond curing diseases. This invites scrutiny into the ethical boundaries of medical intervention, making conversations about access, affordability, and long-term effects necessary as we explore this new capability.
Ethical Considerations in Gene Editing
The discussion surrounding gene editing, especially regarding CRISPR, is heavily steeped in ethics. As highlighted by Baer’s remarks during the Science Center talk, the ability to ‘edit’ our genetic makeup poses significant moral questions. Should interventions be made in cases of genetic conditions that are not life-threatening? As gene editing becomes more prominent, determining who should make these choices—scientists, parents, or society—becomes a matter of profound importance. Baer’s question about editing traits for non-threatening conditions, like Down syndrome, exemplifies the potential slope into eugenics and the alterations that might reshape societal standards.
Equity is another important aspect to consider. The high cost associated with therapies developed from CRISPR technology, such as the sickle cell ‘cure’ priced at $2.2 million, raises concerns about access. If such innovations primarily benefit the affluent, the divisions in health equity will broaden, fostering a society where only the privileged can afford life-saving treatments. Conversations need to include perspectives on fair distribution and the policies required to ensure equitable access to cutting-edge medical advancements.
Health Equity and Genetic Modification Risks
The intersection of genetic modification technology and health equity presents a complex landscape that deserves critical examination. Innovations like CRISPR gene editing promise miraculous healing, yet they also underscore the disparities that exist within healthcare systems. Baer’s concerns about who can afford CRISPR therapies remind us that technological progress should also be accompanied by discussions on socio-economic disparities. Without proactive measures to ensure all populations have access, we risk exacerbating existing health inequities.
Additionally, the risks associated with genetic modification extend beyond financial burdens; they encompass potential health ramifications. The long-term effects of editing germline genes remain largely unknown, igniting fears about unforeseen consequences that may arise from tampering with the fundamental building blocks of life. Reflecting upon Baer’s highlighted interactions among genes, it is crucial to approach CRISPR advancements with caution and a robust regulatory framework that prioritizes both safety and ethics in genetic interventions.
Germline Editing Concerns
Germline editing, or making changes to the genome in sperm, eggs, or embryos, raises unique concerns that transcend individual experiences. While the potential for eradicating genetic diseases exists—leading to healthier future generations—the possibility of creating ‘designer babies’ remains an unsettling facet of this technology. Baer emphasizes the importance of accountability in decision-making related to gene editing; decisions made today could have ramifications for countless lives, influencing traits and conditions that extend far beyond current knowledge.
The ethical implications surrounding germline editing compel us to question the extent to which we should exert control over human biology. When parents consider genetic modifications to confer advantages upon their children, it raises concerns about social norms and pressures. Are we venturing into realms that should be governed by nature rather than human judgment? Deliberations about germline editing should engage not just scientists but also ethicists, policymakers, and the public to navigate this intricate territory responsibly.
Health Justice and Innovative Technologies
As innovative technologies like CRISPR emerge, they bring forth significant health justice implications that cannot be ignored. The notion articulated by Brendel regarding the disparity in accessibility to such innovations echoes a broader concern surrounding the equitable distribution of emerging health technologies. Without an inclusive dialogue that encompasses diverse voices, there is a risk that advancements will favor the affluent, leaving marginalized communities behind.
This reality invites a call to action for healthcare leaders and policymakers alike. It is imperative to foster conversations about health equity that extend beyond mere access to medical technologies. Policymakers must ensure that any novel treatments arising from advancements in gene editing are deployed in a manner that prioritizes fairness and justice across all societal layers.
Long-term Implications of Gene Editing
While CRISPR technology holds the promise of altering the trajectory of genetic diseases, the long-term implications of these interventions remain largely speculative. Baer raises pertinent points about gene interactions, emphasizing that altering one gene could influence numerous processes, potentially leading to unintended health consequences. This underscores the need for comprehensive longitudinal studies that can adequately assess the broader impacts of gene editing on human health and genetic variability.
Investing in research that focuses on the implications of gene editing is essential not only for patient safety but also for establishing informed guidelines in the medical community. The evolving understanding of genetics calls for adaptability in regulations, as findings from gene editing procedures may reshape our understanding of human health over time.
The Role of Oversight in Gene Editing
As highlighted during the presentation, oversight in gene editing practices remains a pressing concern. Despite the legal restrictions in place surrounding germline editing, monitoring practices across various countries remain inconsistent. The potential for unsanctioned practices in regions with lax regulations raises alarm about exploitation and unethical applications of CRISPR technology. Baer’s remarks concerning the monitoring of international gene editing highlight the varying willingness of governments to address these ethical concerns.
Strengthening oversight mechanisms is crucial for safeguarding public health and ethical standards in gene editing. A multi-national cooperative approach could lead to the establishment of universal guidelines that regulate germline editing and experimental gene therapies. This could mitigate the risk of a ‘race to the bottom’ where the lack of regulation breeds unsafe practices and unethical interventions.
Navigating the Future of CRISPR Technology
As we stand on the brink of a new era in medicine with CRISPR technology, navigating the future involves addressing the multifaceted challenges it brings. The ongoing dialogues surrounding ethical considerations, health equity, and long-term implications of gene editing must inform future practices and policies. A balanced approach that embraces innovation while prioritizing ethical standards is essential.
Healthcare leaders, scientists, and ethicists must collaborate to create frameworks that ensure the responsible use of CRISPR. This future must involve public discourse that makes space for diverse perspectives, fostering an environment where advancements in genetics reflect societal values and ethical considerations. We are at a pivotal moment where embracing both technology and ethical responsibility can sculpt a healthier, more equitable future.
Frequently Asked Questions
What are the ethical concerns surrounding CRISPR gene editing?
CRISPR gene editing raises several ethical concerns, including the implications of germline editing, which could allow parents to select desired traits for their children. This brings forth questions about parental authority in making such decisions and the moral implications of altering human genetics. Additionally, the potential for health inequities is a significant concern, as access to CRISPR technology may be limited to wealthier populations, creating disparities in health outcomes.
Can CRISPR gene editing cure sickle cell disease, and what are the costs involved?
Yes, CRISPR gene editing has demonstrated potential to cure sickle cell disease by modifying somatic cells to eliminate the genetic mutation responsible for the condition. However, the treatment comes at a substantial cost of approximately $2.2 million, raising concerns about who can afford the therapy and the equity of access among different populations.
What are the risks associated with genetic modifications through CRISPR technology?
Genetic modifications using CRISPR technology carry risks, including unintended genetic alterations that could lead to unforeseen health issues. Since genes interact in complex ways, editing one gene might disrupt others, potentially causing harmful side effects. It is essential to conduct thorough research and oversight to mitigate these genetic modification risks.
How does germline editing concern impact CRISPR gene editing discussions?
Germline editing concerns significantly impact discussions about CRISPR gene editing, as altering genes in embryos not only affects the individual but also future generations. This raises ethical questions about eugenics, the definition of health, and societal norms regarding disabilities, prompting debate about what constitutes necessary versus elective modifications.
What is the relationship between CRISPR gene editing and health equity?
The introduction of CRISPR gene editing technologies highlights the issue of health equity, as access to these advanced treatments is often limited to those with financial means. Discussions surrounding health equity focus on ensuring that all populations, regardless of socioeconomic status, can benefit from gene editing advancements, thereby preventing further health disparities.
| Key Points | Details |
|---|---|
| CRISPR Technology | CRISPR allows editing of somatic and germline genes, offering potential cures for genetic diseases like sickle cell anemia. |
| Ethical Questions | The use of CRISPR raises ethical issues, such as whether we should edit genes for conditions compatible with life, like Down syndrome. |
| Cost Implications | Gene therapies can be extremely costly; the cure for sickle cell anemia is about $2.2 million, raising questions about accessibility and equity. |
| Health Justice | Disparities may increase due to innovation; focus on ethics and health justice is essential to ensure equitable access to gene editing. |
| Unintended Consequences | Gene modifications may have unexpected effects on overall health, as genes interact in complex ways. |
| Oversight Issues | Concerns about monitoring the use of gene editing technology globally, particularly in countries with less regulation. |
Summary
CRISPR gene editing is at the forefront of revolutionary medical advances, offering potential cures for severe genetic conditions like sickle cell anemia. However, with such advancements come significant ethical dilemmas and cost implications, raising questions about equity and health justice in access to these treatments. As with any powerful technology, careful consideration of the broader impacts on society and individual rights is essential as we navigate the future of gene editing.