Patient safety in medical research is a pivotal concern that underlines the ethical framework governing clinical studies. As our understanding of diseases advances, the integrity of medical research ethics ensures that the rights and well-being of participants remain safeguarded. The recent disruptions in federal research funding, particularly affecting institutions like Harvard, underscore the critical role of IRB safety oversight in maintaining rigorous safety standards. Without adequate resources, crucial assessments required for protecting participants, including thorough risk evaluations and informed consent processes, may be compromised. Hence, robust governance structures, such as those supported by NIH patient protection guidelines, are essential for fostering trust and advancing safe medical innovation through clinical trial oversight.
The realm of clinical studies is intricately linked with the assurance of participant well-being and proper ethical conduct. Safeguarding individuals involved in human health research is vital, as it reflects on the broader principles of medical integrity and accountability. Recent challenges surrounding federal funding restrictions highlight the importance of oversight mechanisms like institutional review boards, which play a crucial role in evaluating research proposals and ensuring ethical compliance. Furthermore, effective governance helps mitigate potential risks and promotes a culture of safety and trust within the research community. In essence, maintaining high standards of patient safety in medical experimentation is not only an ethical imperative but also essential for the advancement of science and public confidence in healthcare research.
The Impact of Funding Cuts on Patient Safety in Medical Research
The recent halt in federal research funding has raised serious concerns regarding patient safety in medical research. Without adequate financial resources, institutions like Harvard are forced to suspend critical oversight mechanisms that protect the welfare of participants. For example, the SMART IRB system plays a vital role in ensuring that diverse and multi-site research studies comply with ethical standards. When funding is cut, these systems struggle to operate effectively, creating gaps in patient protection that could potentially expose participants to various risks.
Moreover, funding reductions inevitably lead to increased bureaucracy and delays in the initiation of new studies. Without the support necessary for comprehensive oversight, Institutional Review Boards (IRBs) may not have the capacity to thoroughly critique research proposals, thereby compromising the safety protocols intended to safeguard participants. The consequences of these disruptions are profound, especially for vulnerable populations who depend on the assurance that their health and rights are protected during medical trials.
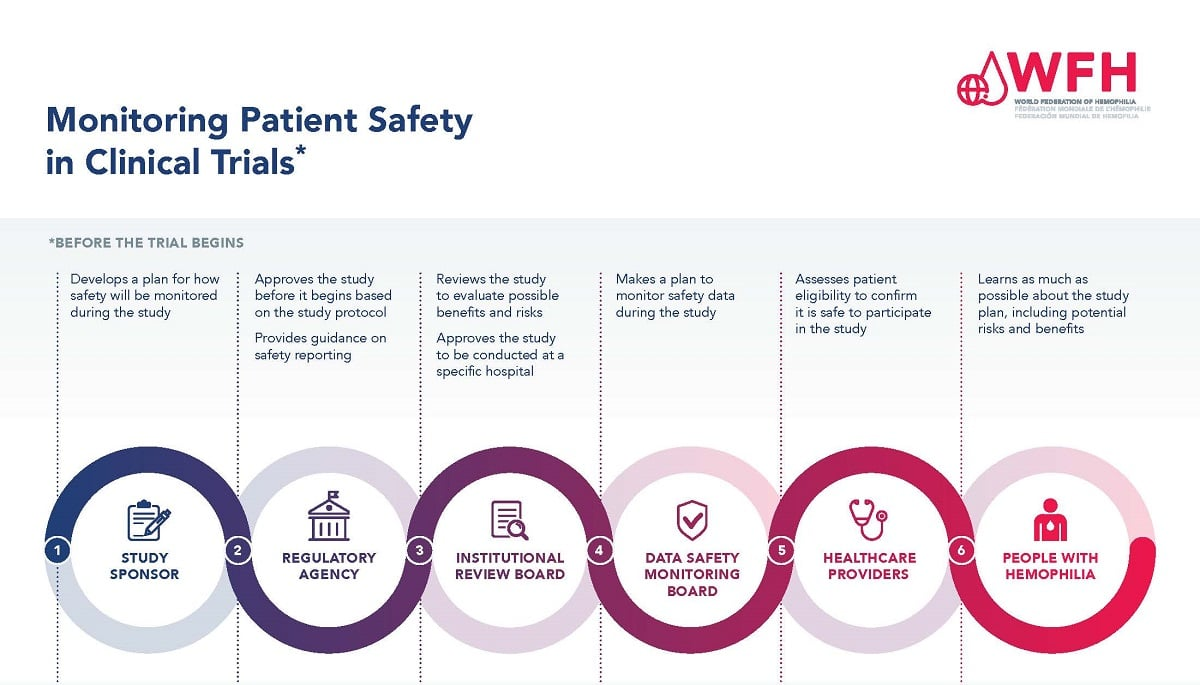
Understanding the Role of Institutional Review Boards (IRBs) in Patient Safety
Institutional Review Boards (IRBs) are fundamental to the ethical conduct of medical research involving human subjects. These boards are responsible for reviewing research proposals to ensure that they minimize risks to participants while maximizing potential benefits. IRBs conduct a detailed analysis of the study design, participant recruitment strategies, and the informed consent process, ensuring that participants are well-informed and protected throughout the research journey.
Furthermore, IRBs play a crucial role in ongoing surveillance during studies, monitoring adverse events and implementing necessary modifications to protect participant safety. This level of oversight helps maintain public trust in the research enterprise. Historical examples of research misconduct underscore the importance of this role, revealing how the lack of stringent oversight can lead to ethical violations and harm to participants. With the current funding cuts, the efficacy of IRBs may be compromised, raising pressing concerns about the safety of those who volunteer for these critical studies.
The Importance of Federal Research Funding for Ethical Oversight
Federal research funding is essential for maintaining robust ethical oversight in medical research. It supports the operations of IRBs, which are tasked with reviewing research proposals and ensuring compliance with regulations that protect human subjects. The National Institutes of Health (NIH) funding, in particular, underpins a significant portion of the research conducted across the United States, allowing for a streamlined approach to multicenter studies through mechanisms like the SMART IRB system.
The recent cuts in federal funding, amounting to over $2 billion, threaten this essential framework, potentially leading to fewer resources for ethical reviews and oversight. Consequently, researchers may find themselves working without the necessary checks and balances in place, increasing the risk to participants’ safety and well-being. This situation can foster skepticism and hinder medical innovation, illustrating the critical interconnectedness of funding, oversight, and the ethical conduct of research.
Ensuring Informed Consent in Research: A Critical Component of Patient Safety
Informed consent is a cornerstone of ethical research practices, ensuring that participants are fully aware of the risks, benefits, and procedures associated with a study. It is the responsibility of IRBs to review and approve the consent forms used in clinical trials, ensuring that they meet ethical standards and transparency requirements. This process is invaluable for safeguarding patient autonomy and protecting participants from potential exploitation or harm.
However, disruptions in funding can significantly impact the resources available for developing effective informed consent protocols. With fewer resources, institutions may struggle to accurately convey complex information, leaving participants in the dark about important aspects of their involvement in research. As a result, ensuring informed consent becomes a challenging endeavor, possibly compromising the trust that is essential for successful research relationships.
The Vital Intersection of Ethics and Medical Research Funding
Ethics in medical research is an ongoing evolution that adapts to historical lessons and societal expectations regarding patient safety. The framework established by prior atrocities in research has necessitated stringent oversight to safeguard participants. However, when federal funding is cut, institutions may face challenges in maintaining these ethical standards. The relationship between funding and ethical oversight is critical, as financial resources translate into the capability of conducting comprehensive reviews and ongoing monitoring.
Without a well-funded research infrastructure, ethical breaches could occur more frequently, undermining the principles of respect for persons, beneficence, and justice that are foundational to research ethics. This funding crisis has the potential to erode public confidence in medical research, making it imperative for stakeholders to advocate for sustained financial support that upholds patient safety and ethical standards.
Addressing Public Trust through Ethical Research Practices
Public trust is paramount for the success of medical research, as the willingness of individuals to participate in studies often hinges on their confidence in the ethical standards upheld by researchers. This trust is maintained through transparent processes, robust oversight, and the assurance that participant safety is a priority. When funding cuts threaten the resources allocated to ethical oversight, the integrity of research practices may come under scrutiny, jeopardizing public confidence.
Institutions must work diligently to rebuild and maintain trust by demonstrating their commitment to ethical research practices and patient safety. Engaging with communities, providing clear communication about research protocols, and ensuring a responsive oversight system are critical steps in fostering that trust. When individuals feel respected and assured of their rights and safety, they are more likely to engage in medical research, thus advancing scientific knowledge.
The Future of Clinical Trials: Navigating Challenges in Patient Protection
As the landscape of medical research evolves, clinical trials face increasingly complex challenges in protecting patient safety. The cutbacks in federal funding impede the ability of institutions to navigate these challenges effectively. In the absence of sufficient resources, IRBs may struggle to keep up with the demands of modern clinical trials, which often involve innovative therapies and diverse participant populations. This could lead to challenges in risk assessment and management, ultimately affecting participant outcomes.
Moreover, as clinical trials adapt to new regulatory environments and technological advancements, the need for robust oversight becomes even more critical. Ensuring that clinical trials adhere to ethical standards while also addressing patient safety concerns will require ongoing investment and support from federal funding agencies. Institutions must remain proactive in seeking alternative funding sources and initiatives that prioritize the health and safety of participants in the evolving research landscape.
Stakeholder Collaboration: Ensuring Comprehensive Patient Safety
Collaboration among stakeholders is crucial for fostering comprehensive patient safety in medical research. This includes partnerships between academic institutions, funding agencies, and community representatives, all of whom have a vested interest in ensuring ethical research practices. Federal research funding not only supports research initiatives but also facilitates dialogue and cooperation among these stakeholders to develop best practices for patient protection.
When stakeholders come together, they can share insights and resources that enhance the effectiveness of oversight mechanisms like IRBs. This collaborative approach builds a stronger ethical framework that prioritizes patient welfare, ensuring that all voices are heard in the research process. By engaging the community and incorporating diverse perspectives, the research enterprise can enhance its commitment to safeguarding the rights and safety of study participants.
Historical Lessons: Shaping the Future of Medical Research Ethics
The history of medical research is fraught with cautionary tales that illuminate the critical importance of ethics and oversight. Historical abuses, such as the Tuskegee syphilis study and the Nazi medical experiments, have necessitated the creation of stringent ethical guidelines and oversight structures like IRBs. These lessons serve as a reminder of the potential consequences of neglecting patient safety and informed consent.
As we look to the future of medical research, these historical rulings must inform ongoing efforts to enhance ethical standards and patient protections. It is essential that funding agencies recognize the importance of these lessons and invest in programs that reinforce the ethical foundations upon which modern research is built, ensuring that patients remain at the forefront of research initiatives.
Frequently Asked Questions
What is the importance of patient safety in medical research?
Patient safety in medical research is crucial as it ensures the health and welfare of individuals participating in clinical studies. Effective oversight from institutional review boards (IRBs) is essential to uphold medical research ethics, protect participants’ rights, and mitigate risks associated with research activities. By maintaining strict safety protocols, researchers can help prevent adverse events and foster public trust in medical research.
How do IRBs enhance patient safety in medical research?
Institutional Review Boards (IRBs) play a vital role in enhancing patient safety in medical research by carefully reviewing study proposals to ensure compliance with medical research ethics. They assess the risks and benefits associated with studies, oversee the informed consent process, and monitor the safety of participants throughout the research lifecycle. Their oversight is fundamental in maintaining the integrity and ethical standards of clinical trials.
How does federal research funding support patient protection in medical studies?
Federal research funding, particularly through agencies like the NIH, supports patient protection by providing resources for the ethical conduct and oversight of clinical research. This funding allows institutions to implement rigorous safety measures, ensure comprehensive IRB review, and conduct studies that prioritize the health and rights of participants, thereby reinforcing patient safety in medical research.
What happens to patient safety when funding for medical research is cut?
Cuts to funding for medical research can severely impact patient safety by disrupting IRB oversight and halting ongoing studies. This can lead to delays in implementing important safety protocols, reducing the capacity to conduct thorough patient monitoring, and increasing the risk of adverse events. Funding cuts can create a ripple effect, undermining public trust in research and compromising the welfare of research participants.
What is the role of SMART IRB in safeguarding patients during medical research?
SMART IRB is instrumental in safeguarding patients during medical research as it provides a streamlined system for IRB review across multiple research sites. This collaborative approach reduces barriers to conducting ethical research while ensuring that patient safety is prioritized through rigorous oversight. By adopting a single IRB model, SMART IRB enhances coordination, improves compliance with medical research ethics, and facilitates quicker, safer studies.
Why is informed consent vital for patient safety in clinical trials?
Informed consent is vital for patient safety in clinical trials because it ensures participants are fully aware of the research objectives, potential risks, and benefits before agreeing to partake. This process is a fundamental aspect of medical research ethics and safeguards participants’ autonomy, empowering them to make informed decisions about their involvement in studies that could impact their health and wellbeing.
How do historical events shape current practices in patient safety in medical research?
Historical events, such as unethical studies and medical experiments, have significantly shaped current practices in patient safety in medical research. These events highlighted the dire need for stringent ethical standards and IRB oversight to protect participants. Today’s regulations and ethical guidelines are a direct response to past abuses, ensuring that patient safety remains a top priority in all research activities.
What are the potential consequences of sacrificing patient safety for research expediency?
Sacrificing patient safety for research expediency can lead to severe consequences, including increased instances of adverse events, loss of public trust in medical research, and impaired health outcomes for participants. It undermines the ethical foundation of research and jeopardizes the integrity of clinical trials, ultimately hindering advancements in medicine and patient care.
| Key Point | Description |
|---|---|
| Funding Cuts Impact | The freeze of over $2 billion in federal research grants disrupts efforts to ensure patient safety in medical research. |
| Role of IRBs | Institutional Review Boards (IRBs) oversee research compliance to protect participant rights and safety, reviewing study designs, consent processes, and risk assessments. |
| Historical Context | Past ethical violations in medical research have shaped the current oversight system to safeguard participants. |
| Community Impact | Funding cuts can erode public trust and reduce research collaboration, potentially delaying innovations in medical treatments. |
| Continued Efforts | Despite setbacks, Harvard Medical School is providing support to maintain essential collaborative research efforts for patient safety. |
Summary
Patient safety in medical research is critically affected by federal funding cuts. The suspension of over $2 billion in funding has created significant challenges for research institutions like Harvard, disrupts oversight systems, and threatens the welfare of participants in medical studies. This highlights the vital role of Institutional Review Boards (IRBs) in protecting participants and ensuring ethical standards are upheld. Without adequate funding and cooperation, the necessary checks and balances that safeguard research participants could be compromised, leading to increased risks and reduced public trust in medical research. It is imperative that stakeholders advocate for robust funding mechanisms to continue supporting patient safety in medical research.